Amides
New Methods for Amide Synthesis
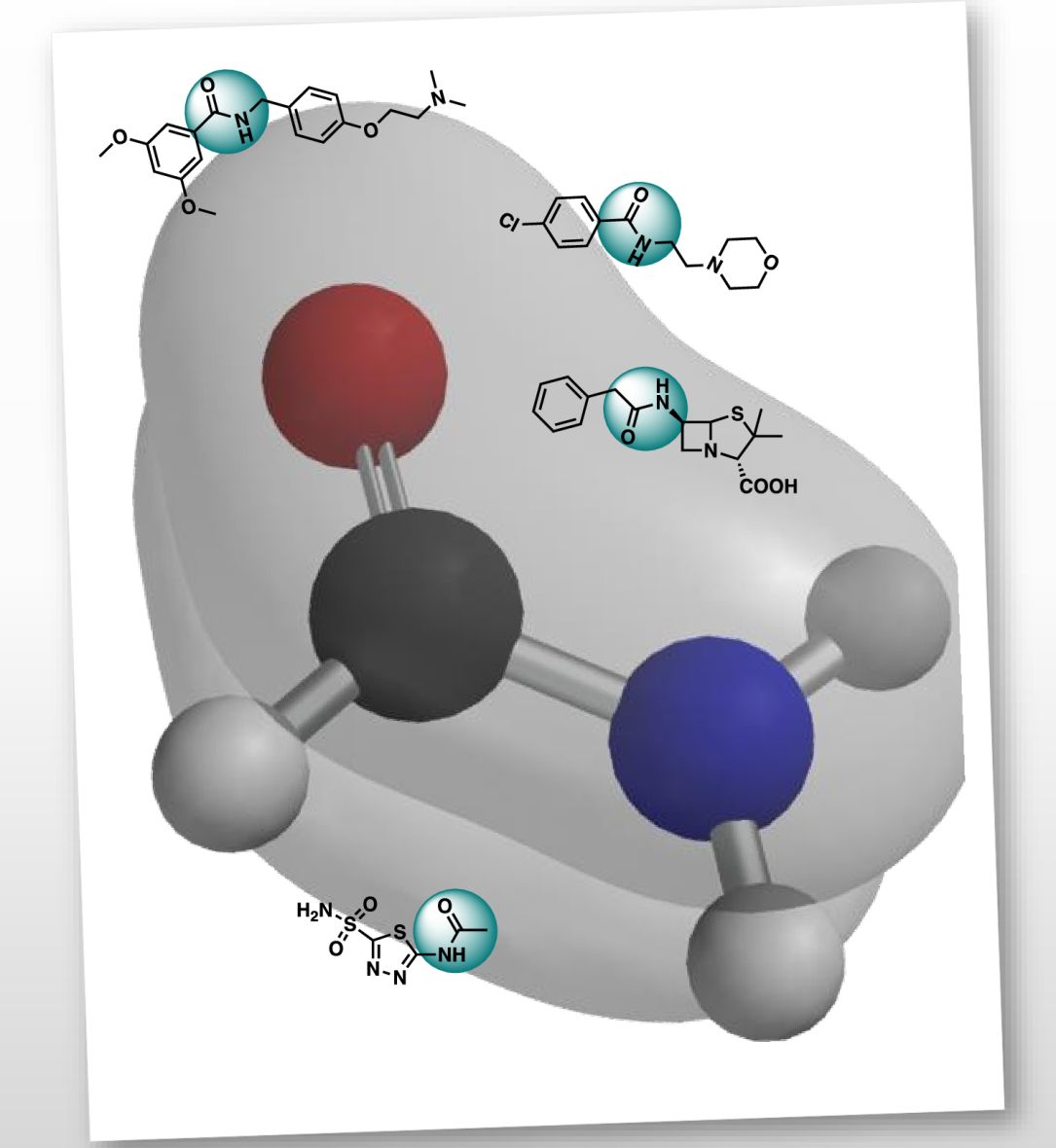
Amides are undeniably one of the most important functional groups in organic chemistry; they are present in many naturally occurring molecules, synthetic polymers, peptides, and pharmaceutical agents, among others.
Current Projects
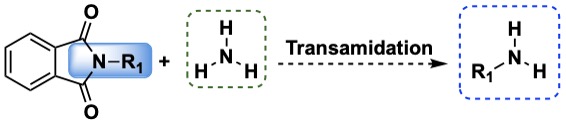
Phtalimide Cleavage
Phthaloyl group has been used in protection of primary amines, its cleavage uses strong reaction conditions and toxic materials. In that context transamidation is a useful alternative to cleave the phthaloyl group using soft reactions conditions.
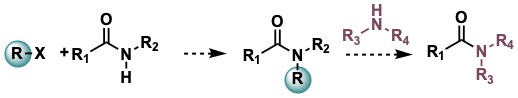
Transamidation of Secondary Amides
The use of twisted amides to increase the electrophilic character of the C=O(N) bond has been studied by several research groups. Subsequent reactions of the amide bond, including transamidation produce significant amounts of residues. Our research focuses on the development of atom economic processes of transamidation of activated secondary amides.
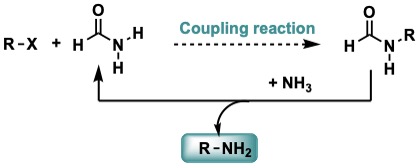
Synthesis of Primary Amines
Amines, produced by a transamidation exchange, are usually discarded, or seen as residues. Our team works in the connection of transamidation reaction and cross-coupling reactions to synthesize complex primary amines.
Previous Results
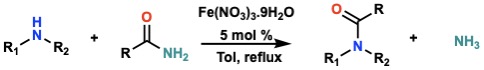
Fe (III)/Water: A Suitable Catalyst for Transamidation
The highly efficient transamidation of several primary, secondary, and tertiary amides with aliphatic and aromatic amines (primary and secondary) was described. The reaction is performed in the presence of 5 mol % of different hydrated salts of Fe(III), and the results show that the presence of water is crucial. The methodology was also applied to urea and phthalimide demonstrating its versatility and wide substrate scope.
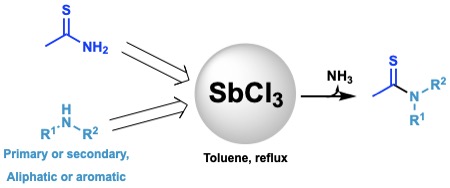
Transamidation of Thioamides
A transamidation reaction of thioacetamide with primary and secondary amines was described. The use of catalytic amounts of SbCl3 notably increases the yields and diminishes the reaction times.
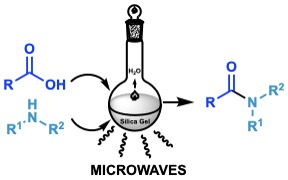
Direct Amidation in Solid Support
A highly improved and green methodology for the direct amidation of carboxylic acids with amines using silica gel as a solid support and catalyst was described. The scope of this method is exemplified using several aliphatic, aromatic, unsaturated and fatty acids. The reaction is also applied to different primary and secondary amines.