Chemistry of Sulfonium Salts and Thionium Ions
We have studied the behavior of chlorosulfoniums and succeeded in their application in new methodologies to obtain simple molecules, but also, we have shown the huge functional group tolerance of these compounds and their potential as intermediates.
Current Projects
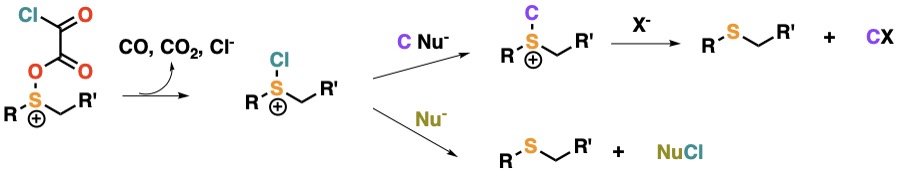
Chemistry of Chlorosulfonium Salts
Activating sulfoxides with oxalyl chloride we obtain chlorosulfonium salts, they may react as source of electrophilic chlorine or in the electrophilic sulfur yielding new sulfoniums with three electrophilic positions.
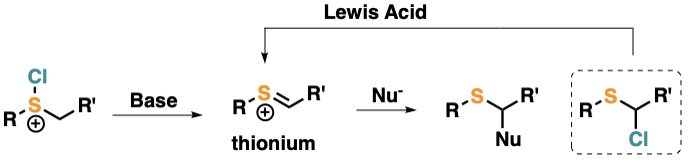
Chemistry of Thionium Ions
We generate thionium ions from chlorosulfonium salts, they usually react with chloride yielding α-chlorosulfides. The use of Lewis acids regenerates the thionium ion which reacts with carbon nucleophiles in cyclization reaction.
Previous Results
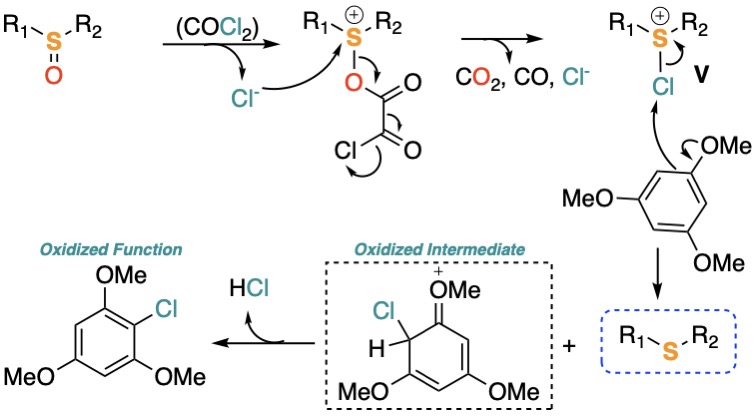
Reduction of Sulfoxides
We described a selective late-stage deoxygenation of sulfoxides based on a novel application of chlorosulfonium salts and, conceptually, a new process using them generated in situ from sulfoxides as the source of electrophilic chlorine. The use of highly nucleophilic 1,3,5-trimethoxybenzene (TMB) as the reducing agent is described for the first time and applied successfully in the deoxygenation of simple and functionalized sulfoxides.
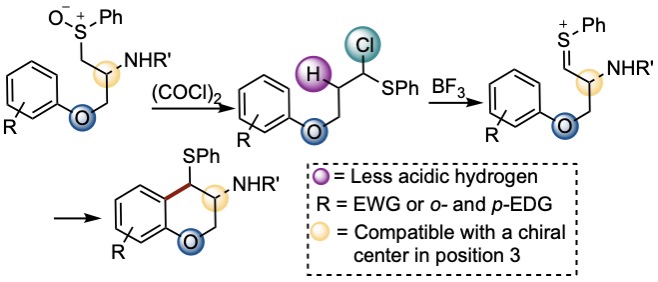
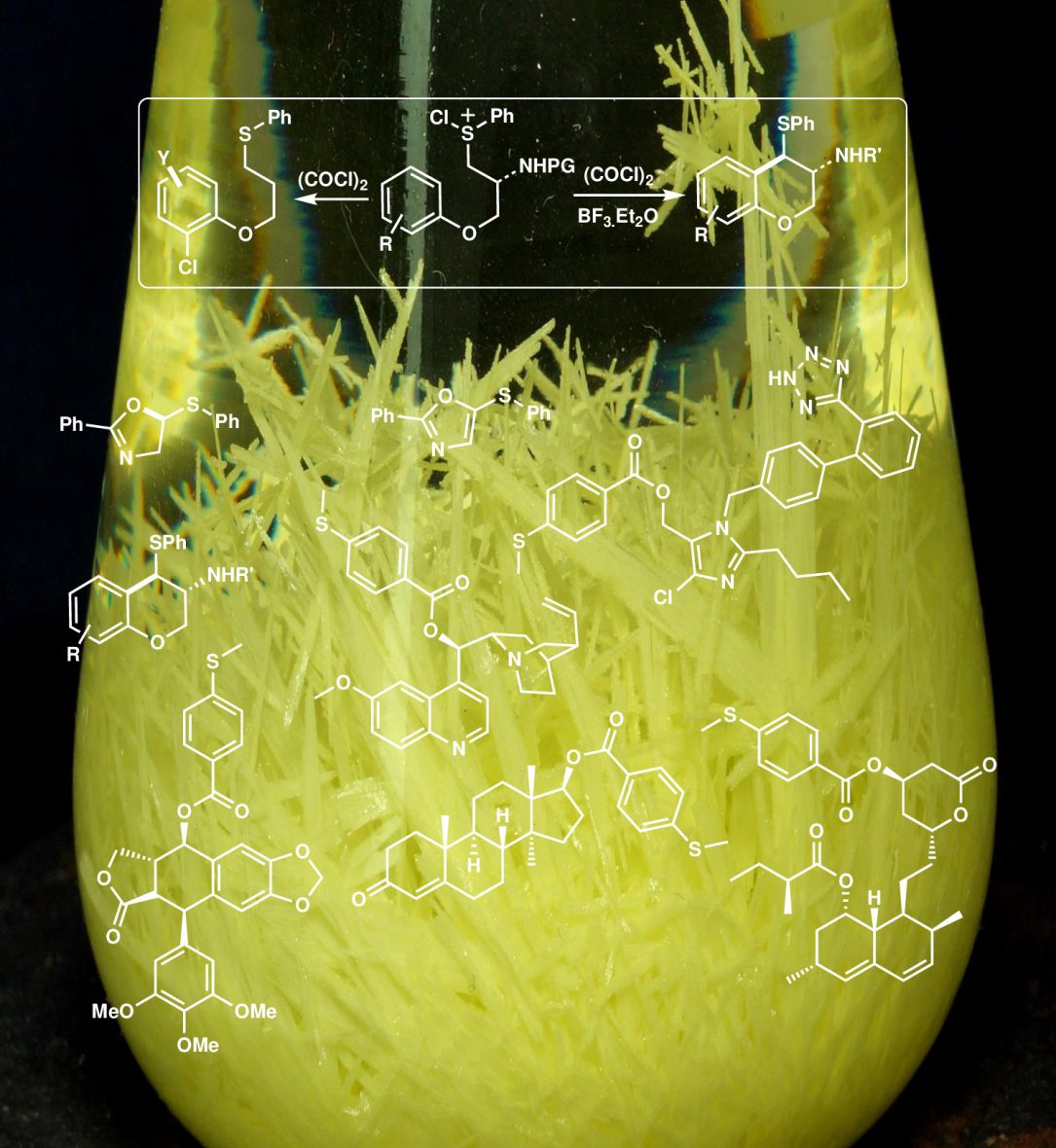
Synthesis of Chromanes
The use of (COCl)2 as Pummerer activator showed substantial activity producing α-chlorinated sulfides that can undergo Pummerer-Friedel Craft cyclization. If the aromatic ring has EDGs in position 3, the reaction follows a different pathway, yielding the reductive chlorination products where the chlorine atom comes from a sulfonium salt.
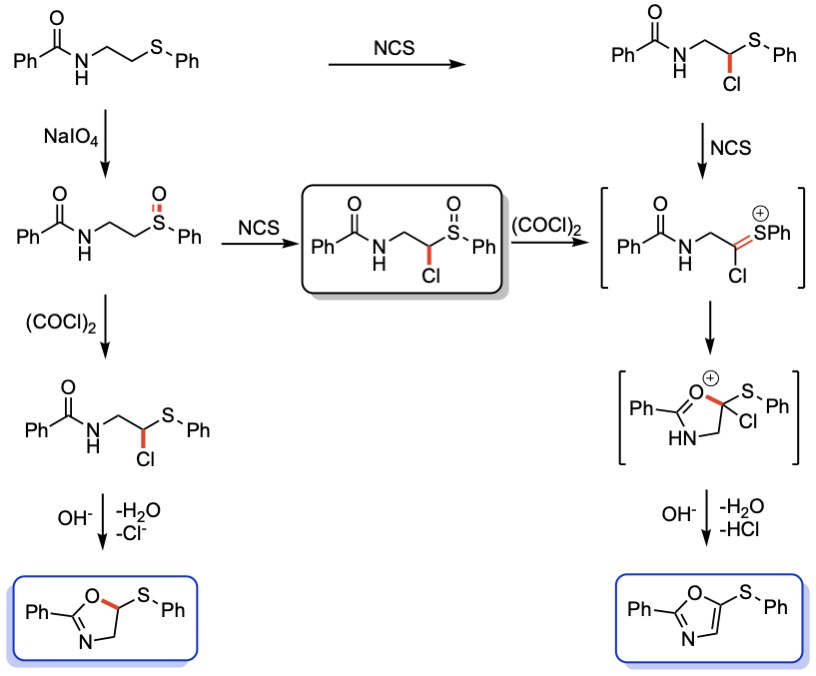
Synthesis of Oxazolines
A rapid and simple method to access unnatural 2-substituted 5-thio oxazolines has been developed. The methodology is based on a Pummerer reaction followed by an intramolecular nucleophilic substitution. The reaction proved to be general, and different substituents, such as alkyl, aryl, alkenyl and functionalized groups, can be used without a significant decrease in efficiency.